Manufacturing and Distribution
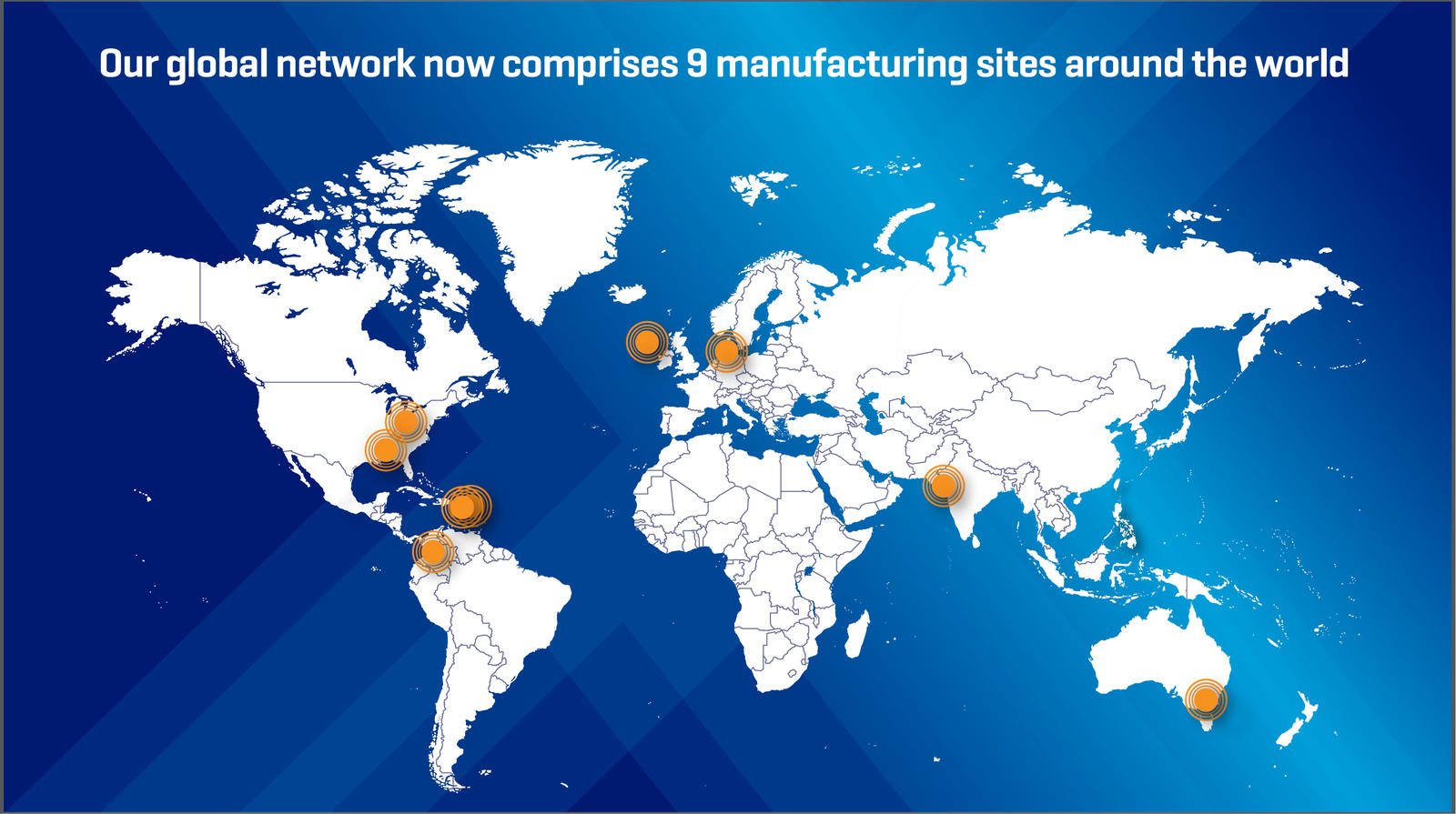
In response to growing healthcare needs, Baxter has invested $1 billion into its manufacturing network in recent years. We continually invest in filling our pipeline with new products to support patient care.
Our Network

Take the Baxter Tour
Baxter invites all its valued customers to tour our state-of-the-art manufacturing plant in Round Lake, Illinois. Please contact your representative directly or click here to arrange a guided tour.
Investment
With ongoing investments in world-class manufacturing, meaningful innovations and a focus on patient safety, Baxter prioritizes patients and healthcare providers by ensuring that innovative treatment, solutions and support are available to help enhance safety and improve patient care.
Baxter has increased the supply of critical products, demonstrating our speed and commitment to responding to market and supply dynamics.

Baxter Partnerships
Baxter continually reviews our suppliers of raw materials and active pharmaceutical ingredients (API), putting redundancies in place for the most critical products to help ensure a steady supply of medications to support patient care.