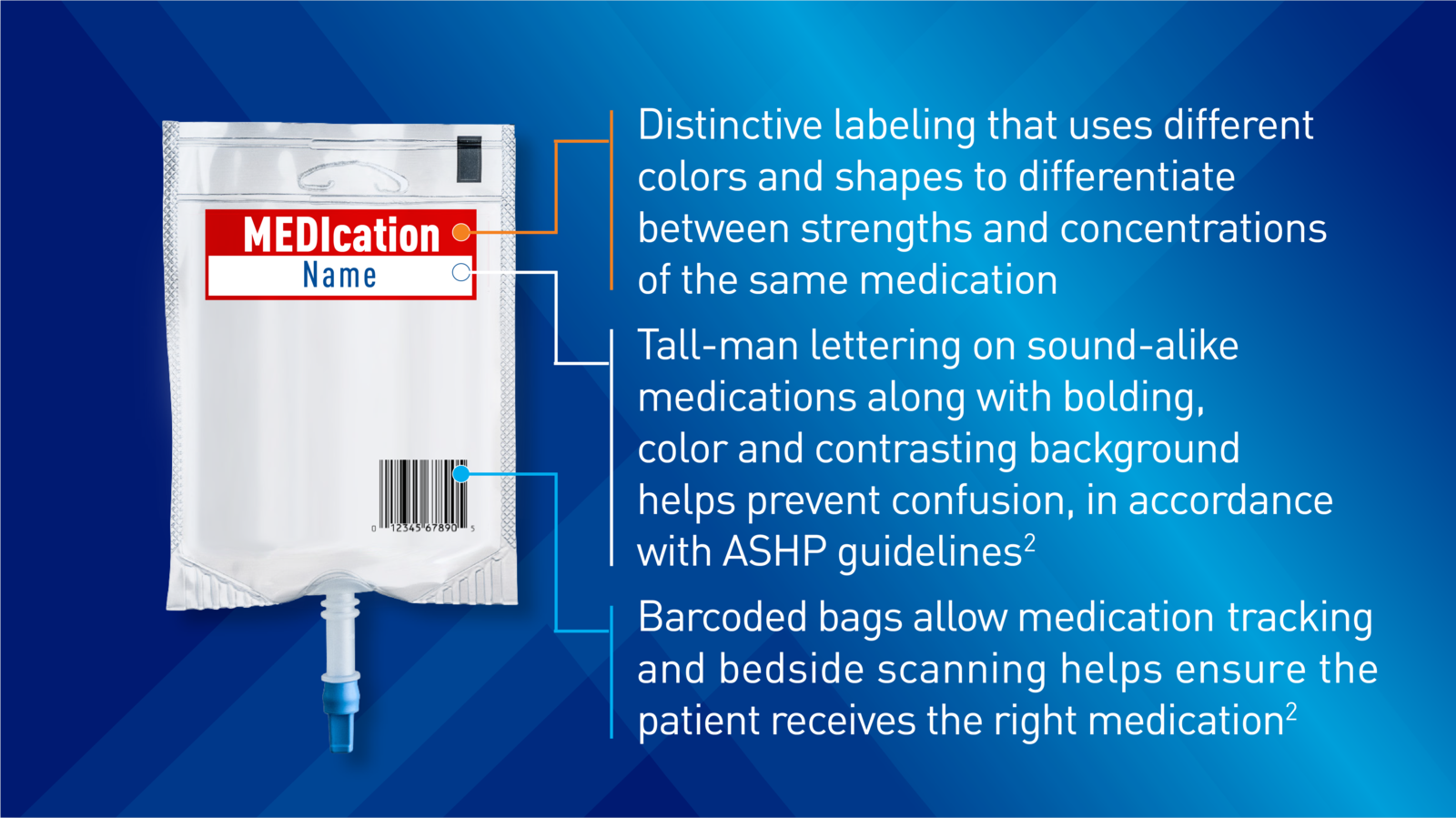
High Alert Ready-to-Use Premix Medications
See Featured Ready-to-Use Products See Frozen Premix ProductsEverywhere patients are treated, Baxter high alert, ready-to-use medications help minimize compounding errors1,2 that may turn routine care into urgent situations. For clinicians who routinely deliver urgent care, Baxter high alert ready-to-use medications may help reduce complexity.
High alert medications are drugs that bear a heightened risk of causing significant patient harm when they are used in error.3
Baxter Helps Meet Joint Commission Standards for Managing High Alert Medications
The Joint Commission requires hospitals to develop their own high alert medication list and implement a plan for their management.4 Baxter offers high alert medications in premix IV formulations, which may help reduce the associated risk related to high alert medications, while supporting your overall safety protocols and helping to increase hospital efficiency.1,2
Consider implementing premix medications in your facility as part of your risk reduction strategy. Commercially prepared premix IV medications are recommended to be used by ISMP and ASHP when available.1,2
Ready to see how Baxter’s High Alert Ready-to-Use Medications can fit into your workflow?
Featured High Alert Medications in Premix Formulations
Baxter High Alert Medications in Premix Formulations
Please click here to see accompanying Indications, Important Risk Information, and link to the Prescribing Information for each of these products below.
Convenient ways to place your order
How might your facility benefit from adding Baxter Pharmaceuticals? Connect with us to learn more.
Please ask your Baxter representative how Baxter premixes can help your healthcare operations.

Wholesaler Services
Order direct through your wholesaler
Dexmedetomidine Hydrochloride in 0.9% Sodium Chloride Injection
200 mcg/50 mL (4 mcg/mL) in a 50 mL Galaxy container
400 mcg/100 mL (4 mcg/mL) in a 100 mL Galaxy container
Indications and Important Risk Information
Indications
Dexmedetomidine hydrochloride in 0.9% sodium chloride injection is an alpha2-adrenergic receptor agonist indicated for:
- Sedation of initially intubated and mechanically ventilated adult patients during treatment in an intensive care setting. Administer dexmedetomidine hydrochloride in 0.9% sodium chloride injection by continuous infusion not to exceed 24 hours.
- Sedation of non-intubated adult patients prior to and/or during surgical and other procedures.
Important Risk Information
- Contraindications: None
- Monitoring: Dexmedetomidine hydrochloride in 0.9% sodium chloride injection should be administered only by persons skilled in the management of patients in the intensive care or operating room setting. Patients should be continuously monitored.
- Hypotension, Bradycardia, and Sinus Arrest: Clinically significant episodes of bradycardia and sinus arrest have been reported with administration in young, healthy adult volunteers with high vagal tone or with different routes of administration including rapid intravenous or bolus administration.
Reports of hypotension and bradycardia have been associated with dexmedetomidine hydrochloride in 0.9% sodium chloride injection infusion. Some of these cases have resulted in fatalities. If medical intervention is required, treatment may include decreasing or stopping the infusion, increasing the rate of intravenous fluid administration, elevation of the lower extremities, and use of pressor agents.
Caution should be exercised when administering to patients with advanced heart block and/or severe ventricular dysfunction. Hypotension and/or bradycardia may be expected to be more pronounced in patients with hypovolemia, diabetes mellitus, or chronic hypertension and in elderly patients.
Use with caution with co-administration with other vasodilators or negative chronotropic agents. - Transient Hypertension: Has been observed primarily during the loading dose. Treatment has generally not been necessary, although reduction of the loading infusion rate may be desirable.
- Arousability: Some patients have been observed to be arousable and alert when stimulated. This alone should not be considered as evidence of lack of efficacy in the absence of other clinical signs and symptoms.
- Tolerance and Tachyphylaxis: Use of dexmedetomidine beyond 24 hours has been associated with tolerance and tachyphylaxis and a dose-related increase in adverse reactions.
- Hyperthermia or Pyrexia: May be resistant to traditional cooling methods, such as administration of cooled intravenous fluids and antipyretic medications. Discontinue if drug-related hyperthermia or pyrexia is suspected and monitor patients until body temperature normalizes.
- Hepatic Impairment: Clearance decreases with severity of hepatic impairment, dose reduction should be considered in patients with impaired hepatic function.
- Adverse Reactions:
- The most common adverse reactions (incidence >2%) in adults are hypotension, bradycardia, and dry mouth.
- Adverse reactions in adults, associated with infusions >24 hours in duration include ARDS, respiratory failure, and agitation.
- Drug Interactions:
- Anesthetics, Sedatives, Hypnotics, Opioids: Enhancement of pharmacodynamic effects. Reduction in dosage of dexmedetomidine hydrochloride in 0.9% sodium chloride injection or the concomitant medication may be required.
Please see accompanying full Prescribing Information for Dexmedetomidine Hydrochloride in 0.9% Sodium Chloride Injection.
Dobutamine Hydrochloride in 5% Dextrose Injection
250 mg/250 mL, 500 mg/250 mL, 1000 mg/250 mL
Indications and Important Risk Information
Indications
Dobutamine Hydrochloride in 5% Dextrose Injection is indicated when parenteral therapy is necessary for inotropic support in the short-term treatment of patients with cardiac decompensation due to depressed contractility resulting either from organic heart disease or from cardiac surgical procedures. Experience with intravenous dobutamine in controlled trials does not extend beyond 48 hours of repeated boluses and/or continuous infusions.
Whether given orally, continuously intravenously, or intermittently intravenously, neither dobutamine nor any other cyclic-AMP-dependent inotrope has been shown in controlled trials to be safe or effective in the long-term treatment of congestive heart failure.
Important Risk Information
Contraindications
Dobutamine Hydrochloride in 5% Dextrose Injection is contraindicated in patients with idiopathic hypertrophic subaortic stenosis and in patients who have shown previous manifestations of hypersensitivity to dobutamine.
Solutions containing dextrose may be contraindicated in patients with known allergy to corn or corn products.
Warnings and Precautions
- Increase in Heart Rate or Blood Pressure: Dobutamine Hydrochloride in 5% Dextrose Injection may cause a marked increase in heart rate or blood pressure, especially systolic pressure. Usually, reduction of dosage reverses these effects. Because dobutamine facilitates atrioventricular conduction, patients with atrial fibrillation are at risk of developing rapid ventricular response. Patients with preexisting hypertension appear to face an increased risk of developing an exaggerated pressor response. In patients who have atrial fibrillation with rapid ventricular response, a digitalis preparation should be used prior to institution of therapy with Dobutamine in D5W.
- Ectopic Activity: Dobutamine Hydrochloride in 5% Dextrose Injection may precipitate or exacerbate ventricular ectopic activity, but it rarely has caused ventricular tachycardia.
- Hypersensitivity: Reactions suggestive of hypersensitivity associated with administration of dobutamine including skin rash, fever, eosinophilia, and bronchospasm, have been reported occasionally. Dobutamine Hydrochloride in 5% Dextrose Injection contains sodium bisulfite, a sulfite that may cause allergic-type reactions, including anaphylactic symptoms and life-threatening or less severe asthmatic episodes, in certain susceptible people. The overall prevalence of sulfite sensitivity in the general population is unknown and probably low. Sulfite sensitivity is seen more frequently in asthmatic than in non-asthmatic people.
- General: During the administration of Dobutamine Hydrochloride in 5% Dextrose Injection, as with any adrenergic agent, ECG and blood pressure should be continuously monitored. In addition, pulmonary wedge pressure and cardiac output should be monitored whenever possible to aid in the safe and effective infusion of dobutamine.
Hypovolemia should be corrected with suitable volume expanders before treatment with dobutamine is instituted. No improvement may be observed in the presence of marked mechanical obstruction, such as severe valvular aortic stenosis. - Adverse Reactions:
- Hypotension: Precipitous decreases in blood pressure have occasionally been described in association with dobutamine therapy. Decreasing the dose or discontinuing the infusion typically results in rapid return of blood pressure to baseline values. In rare cases, however, intervention may be required and reversibility may not be immediate.
- Stress Cardiomyopathy: Stress cardiomyopathy has been reported with dobutamine in association with cardiac stress testing.
- Reactions at Sites of Intravenous Infusion: Phlebitis has occasionally been reported. Local inflammatory changes have been described following inadvertent infiltration.
- The following adverse effects have been reported in 1% to 3% of adult patients: nausea, headache, anginal pain, nonspecific chest pain, palpitations, and shortness of breath.
- Drug Interactions: There was no evidence of drug interactions in clinical studies in which dobutamine was administered concurrently with other drugs, including digitalis preparations, furosemide, spironolactone, lidocaine, glyceryl trinitrate, isosorbide dinitrate, morphine, atropine, heparin, protamine, potassium chloride, folic acid, and acetaminophen. Preliminary studies indicate that the concomitant use of dobutamine and nitroprusside results in a higher cardiac output and, usually, a lower pulmonary wedge pressure than when either drug is used alone.
Please see accompanying full Prescribing Information for Dobutamine Hydrochloride in 5% Dextrose Injection.
Dopamine Hydrochloride and 5% Dextrose Injection, USP
800 mcg/mL in 250 mL; 1600 mcg/mL in 250 mL; 800 mcg/mL in 500 mL; 3200 mcg/mL in 250 mL; 1600 mcg/mL in 500 mL
Indications and Important Risk Information
Indications
Dopamine hydrochloride is indicated for the correction of hemodynamic imbalances present in the shock syndrome due to myocardial infarctions, trauma, endotoxic septicemia, open heart surgery, renal failure and chronic cardiac decompensation as in congestive failure.
Where appropriate, restoration of blood volume with a suitable plasma expander or whole blood should be instituted or completed prior to administration of dopamine hydrochloride.
Patients most likely to respond adequately to dopamine hydrochloride are those in whom physiological parameters, such as urine flow, myocardial function and blood pressure have not undergone profound deterioration. Reports indicate that the shorter the time interval between onset of signs and symptoms and initiation of therapy with volume correction and dopamine hydrochloride, the better the prognosis.
Important Risk Information
Contraindications
Dopamine hydrochloride should not be used in patients with pheochromocytoma.
Dopamine hydrochloride should not be administered in the presence of uncorrected tachyarrhythmias or ventricular fibrillation.
Solutions containing dextrose may be contraindicated in patients with known allergy to corn or corn products.
Warnings and Precautions
- Evidence is inadequate for fully defining proper dosage and limitations for use in children.
- Contains sodium bisulfite, a sulfite that may cause allergic-type reactions including anaphylactic symptoms and life-threatening or less severe asthmatic episodes in certain susceptible people. Sulfite sensitivity is seen more frequently in asthmatic than in non-asthmatic people.
- Do not add Dopamine Hydrochloride and 5% Dextrose Injection, USP to any alkaline diluent solution since dopamine hydrochloride is inactivated in alkaline solution.
- Avoid bolus administration of dopamine hydrochloride.
- Avoid Hypovolemia: Prior to treatment with dopamine hydrochloride, hypovolemia should be fully corrected, if possible, with either whole blood, plasma, or plasma expanders as indicated. Monitoring of central venous pressure or left ventricular filling pressure may be helpful in detecting and treating hypovolemia.
- Hypoxia, Hypercapnia, Acidosis: These conditions, which may also reduce the effectiveness and/or increase the incidence of adverse effects of dopamine, must be identified and corrected prior to, and concurrently with, administration of dopamine HCl.
- Ventricular Arrhythmias: If an increased number of ectopic beats is observed, the dose should be reduced if possible.
- Decreased Pulse Pressure: If a disproportionate rise in the diastolic pressure (i.e., marked decrease in the pulse pressure) is observed in patients receiving dopamine hydrochloride, the infusion rate should be decreased and the patient observed carefully for further evidence of predominant vasoconstrictor activity, unless such an effect is desired.
- Hypotension: At lower infusion rates, if hypotension occurs, the infusion rate should be rapidly increased until adequate blood pressure is obtained. If hypotension persists, dopamine HCl should be discontinued and a more potent vasoconstrictor agent such as norepinephrine should be administered.
- Occlusive Vascular Disease: Patients with a history of occlusive vascular disease should be closely monitored for any changes in color or temperature of the skin in the extremities. If a change in skin color or temperature occurs and is thought to be the result of compromised circulation to the extremities, the benefits of continued dopamine hydrochloride infusion should be weighed against the risk of possible necrosis. This condition may be reversed by either decreasing the rate or discontinuing the infusion.
- Extravasation: Dopamine Hydrochloride and 5% Dextrose Injection, USP should be infused into a large vein whenever possible to prevent the possibility of extravasation into tissue adjacent to the infusion site. Extravasation may cause necrosis and sloughing of surrounding tissue. Large veins of the antecubital fossa are preferred to veins in the dorsum of the hand or ankle. Less suitable infusion sites should be used only if the patient’s condition requires immediate attention. The physician should switch to more suitable sites as rapidly as possible. The infusion site should be continuously monitored for free flow.
|
IMPORTANT – Antidote for Peripheral Ischemia
To prevent sloughing and necrosis in ischemic areas, the area should be infiltrated as soon as possible with 10 to 15 mL of 0.9% Sodium Chloride Injection, USP containing from 5 to 10 mg phentolamine, an adrenergic blocking agent. A syringe with a fine hypodermic needle should be used and the solution liberally infiltrated throughout the ischemic area. Sympathetic blockage with phentolamine causes immediate and conspicuous local hyperemic changes if the area is infiltrated within 12 hours. Therefore, phentolamine should be given as soon as possible after the extravasation is noted. |
- Weaning: When discontinuing the infusion, it may be necessary to gradually decrease the dose of dopamine HCl while expanding blood volume with IV fluids, since sudden cessation may result in marked hypotension.
- Adverse Reactions: The following adverse reactions have been observed, but there are not enough data to support an estimate of their frequency: ventricular arrhythmia, atrial fibrillation, ectopic beats, tachycardia, anginal pain, palpitation, cardiac conduction abnormalities, widened QRS complex, bradycardia, hypotension, hypertension, vasoconstriction, dyspnea, nausea, vomiting, azotemia, headache, anxiety, piloerection, gangrene of the extremities.
- Drug Interactions:
- Cyclopropane or halogenated hydrocarbon anesthetics increase cardiac autonomic irritability and may sensitize the myocardium to the action of certain intravenously administered catecholamines, such as dopamine. This interaction may produce ventricular arrhythmias. Therefore, EXTREME CAUTION should be exercised when administering dopamine HCl to patients receiving cyclopropane or halogenated hydrocarbon anesthetics.
- Because dopamine is metabolized by monoamine oxidase (MAO), inhibition of this enzyme prolongs and potentiates the effect of dopamine. Patients who have been treated with MAO inhibitors within two to three weeks prior to the administration of dopamine should receive initial doses of dopamine hydrochloride no greater than one-tenth (1/10) of the usual dose.
- Tricyclic antidepressants may potentiate the cardiovascular effects of adrenergic agents.
- Cardiac effects of dopamine are antagonized by beta-adrenergic blocking agents, such as propranolol and metoprolol.
- The concomitant use of vasopressors, vasoconstrictor agents (such as ergonovine) and some oxytocic drugs may result in severe hypertension.
- Administration of phenytoin to patients receiving dopamine HCl has been reported to lead to hypotension and bradycardia. It is suggested that in patients receiving dopamine HCl, alternatives to phenytoin should be considered if anticonvulsant therapy is needed.
Please see accompanying full Prescribing Information for Dopamine Hydrochloride and 5% Dextrose Injection, USP.
Eptifibatide Injection
75 mg/ 100 mL
Indications and Important Risk Information
Indications
Eptifibatide injection is a platelet aggregation inhibitor indicated for:
- Acute Coronary Syndrome (ACS): Eptifibatide injection is indicated to decrease the rate of a combined endpoint of death or new myocardial infarction (MI) in patients with ACS, including patients who are to be managed medically and those undergoing percutaneous coronary intervention (PCI).
- Percutaneous Coronary Intervention (PCI): Eptifibatide injection is indicated to decrease the rate of a combined endpoint of death, new MI, or need for urgent intervention in patients undergoing PCI, including those undergoing intracoronary stenting.
Important Risk Information
- Contraindications: Treatment with eptifibatide is contraindicated in patients with:
- A history of bleeding diathesis, or evidence of active abnormal bleeding within the previous 30 days
- Severe hypertension (systolic blood pressure >200 mm Hg or diastolic blood pressure >110 mm Hg) not adequately controlled on antihypertensive therapy
- Major surgery within the preceding 6 weeks
- History of stroke within 30 days or any history of hemorrhagic stroke
- Current or planned administration of another parenteral GP IIb/IIIa inhibitor
- Dependency on renal dialysis
- Hypersensitivity to eptifibatide or any component of the product (hypersensitivity reactions that occurred included anaphylaxis and urticaria)
- Bleeding: Bleeding is the most common complication encountered during eptifibatide therapy and is associated with an increase in major and minor bleeding. Most major bleeding associated with eptifibatide has been at the arterial access site for cardiac catheterization or from the gastrointestinal or genitourinary tract. Minimize the use of arterial and venous punctures, IM injections, and the use of urinary catheters, nasotracheal intubation, and nasogastric tubes. When obtaining intravenous access, avoid non-compressible sites.
- Use of Thrombolytics, Anticoagulants, and Other Antiplatelet Agents: Risk factors for bleeding include older age, a history of bleeding disorders, and concomitant use of drugs that increase the risk of bleeding. Concomitant treatment with other inhibitors of platelet receptor glycoprotein (GP) IIb/IIIa should be avoided. In patients treated with heparin, bleeding can be minimized by close monitoring of the aPTT and ACT.
- Care of the Femoral Artery Access Site in Patients Undergoing Percutaneous Coronary Intervention (PCI): In patients undergoing PCI, treatment with eptifibatide is associated with an increase in major and minor bleeding at the site of arterial sheath placement. Heparin use is discouraged after the PCI procedure. Both heparin and eptifibatide should be discontinued and sheath hemostasis should be achieved at least 2 to 4 hours before hospital discharge. If bleeding at access site cannot be controlled with pressure, infusion of eptifibatide and heparin should be discontinued immediately.
- Thrombocytopenia: There have been reports of acute, profound thrombocytopenia (immune-mediated and non-immune mediated) with eptifibatide. In the event of acute profound thrombocytopenia or a confirmed platelet decrease to <100,000/mm3, discontinue eptifibatide and heparin. Monitor serial platelet counts, assess the presence of drug-dependent antibodies, and treat as appropriate. There has been no clinical experience with eptifibatide initiated in patients with a baseline platelet count <100,000/mm3. If a patient with low platelet counts is receiving eptifibatide, their platelet count should be monitored closely.
- Adverse Reactions: Bleeding and hypotension are the most commonly reported adverse reactions.
- Geriatric Use: Risk of bleeding increases with age.
- Pediatric Use: Safety and effectiveness of eptifibatide in pediatric patients have not been studied.
- Drug Interactions: Coadministration of antiplatelet agents, thrombolytics, heparin, aspirin, and chronic NSAID use increases the risk of bleeding. Avoid concomitant use with other glycoprotein (GP) IIb/IIIa inhibitors.
Please see accompanying full Prescribing Information for Eptifibatide Injection.
Heparin Sodium in 0.9% Sodium Chloride Injection
- 1,000 USP units in 500 mL (2 USP units per mL)
- 2,000 USP units in 1000mL (2 USP units per mL)
Indications and Important Risk Information
Indications
- Heparin Sodium in Sodium Chloride Injection at a concentration of 2 units/mL is indicated as an anticoagulant to maintain catheter patency.
Important Risk Information
- Contraindications: Heparin Sodium in Sodium Chloride Injection is contraindicated in patients with the following conditions:
- With an uncontrollable active bleeding state, except when this is due to disseminated intravascular coagulation
- With a history of heparin-induced thrombocytopenia (HIT) or heparin-induced thrombocytopenia with thrombosis (HITT)
- With severe thrombocytopenia
- Known hypersensitivity to heparin or pork products
- Hemorrhage: Avoid using heparin in the presence of major bleeding, except when the benefits of heparin therapy outweigh the potential risks. Hemorrhage can occur at virtually any site in patients receiving heparin. Fatal hemorrhages have occurred. Use heparin sodium with caution in disease states in which there is increased risk of hemorrhage. Monitor for signs of bleeding and manage promptly.
- HIT and HITT: HIT is a serious immune-mediated reaction. HIT may progress to the development of venous and arterial thromboses, a condition referred to as HITT. Monitor any degree of thrombocytopenia closely. Thrombotic events may also be the initial presentation for HITT. These serious thromboembolic events include deep vein thrombosis, pulmonary embolism, cerebral vein thrombosis, limb ischemia, stroke, myocardial infarction, mesenteric thrombosis, renal arterial thrombosis, skin necrosis, gangrene of the extremities that may lead to amputation, and possibly death. If the platelet count falls below 100,000/mm3 or if recurrent thrombosis develops, promptly discontinue heparin, evaluate for HIT and HITT, and, if necessary, administer an alternative anticoagulant. Once HIT or HITT is diagnosed or strongly suspected, discontinue all heparin sources (including heparin flushes) and use an alternative anticoagulant. HIT or HITT can occur up to several weeks after the discontinuation of heparin therapy. Monitor for signs and symptoms and discontinue if indicative of HIT or HITT.
- Hyperkalemia: Heparin can suppress adrenal secretion of aldosterone leading to hyperkalemia, particularly in patients with diabetes mellitus, chronic renal failure, pre-existing metabolic acidosis, a raised plasma potassium, or taking potassium sparing drugs. The risk of hyperkalemia appears to increase with duration of therapy but is usually reversible upon discontinuation. Measure plasma potassium in patients at risk of hyperkalemia before starting heparin therapy and periodically in all patients.
- Elevations of serum aminotransferases: Significant elevations of aspartate aminotransferase (AST) and alanine aminotransferase (ALT) levels have occurred patients who have received heparin. Interpret elevation of these enzymes with caution. These elevations typically resolve upon discontinuation.
- Adverse Reactions: Most common adverse reactions are hemorrhage, thrombocytopenia, HIT and HITT, hypersensitivity, and elevations of aminotransferase levels.
- Drug Interactions: Drugs that interfere with platelet aggregation or drugs that counteract coagulation may induce bleeding.
- Pediatric Use: Safety and effectiveness in pediatric patients have not been established.
- Geriatric Use: A higher incidence of bleeding has been reported in patients over 60 years of age, especially women. Clinical studies indicate that lower doses of heparin may be indicated in these patients.
Please see accompanying full Prescribing Information for Heparin Sodium in 0.9% Sodium Chloride Injection.
Lidocaine Hydrochloride and 5% Dextrose Injection, USP
2 grams/250 mL (8 mg/mL) and 2 grams/500 mL (4 mg/mL)
Indications and Important Risk Information
Indications
Lidocaine Hydrochloride administered intravenously is specifically indicated in the acute management of:
- Ventricular arrhythmias occurring during cardiac manipulations, such as cardiac surgery
- Life-threatening arrhythmias which are ventricular in origin, such as occur during acute myocardial infarction
Important Risk Information
Contraindications
Hypersensitivity reactions, including anaphylactic reactions, have been reported with lidocaine. Lidocaine hydrochloride is contraindicated in patients with a history of hypersensitivity to local anesthetics of the amide type.
Lidocaine is contraindicated in patients with Stokes-Adams syndrome, Wolff-Parkinson- White syndrome, or with severe degrees of sinoatrial, atrioventricular, or intraventricular block.
Warnings and Precautions
- Monitoring: Constant monitoring with an electrocardiograph is essential to the administration of lidocaine hydrochloride intravenously. Signs of excessive depression of cardiac conductivity, such as prolongation of the PR interval, widening of the QRS interval and the appearance or aggravation of arrhythmias, should be followed by prompt cessation of the intravenous infusion of this agent. It is mandatory to have emergency resuscitative equipment and drugs immediately available to manage adverse reactions involving cardiovascular, respiratory, or central nervous systems. Central nervous system adverse reactions are associated with venous plasma levels above 6.0 μg free base per mL.
- Hypersensitivity: Hypersensitivity, including anaphylaxis, has been reported with lidocaine-containing solutions. Stop the infusion immediately if signs of hypersensitivity develop.
- Atrial Fibrillation or Flutter: Acceleration of ventricular rate may occur in patients with atrial fibrillation or flutter treated with lidocaine.
- Sinus bradycardia or incomplete heart block: In patients with sinus bradycardia or incomplete heart block, the administration of lidocaine hydrochloride intravenously for the elimination of ventricular ectopic beats without prior acceleration in heart rate (e.g., by isoproterenol or by electric pacing) may promote more frequent and serious ventricular arrhythmias or complete heart block.
- Renal or hepatic insufficiency: Because lidocaine is metabolized mainly in the liver and excreted by the kidneys, patients with renal or hepatic insufficiency may be at increased risk for toxicity.
- Malignant Hyperthermia: If malignant hyperthermia develops, discontinue administration immediately and institute therapeutic countermeasures as clinically indicated.
- Adverse Reactions: Systemic reactions of the following types have been reported:
Nervous System Disorders: respiratory depression and arrest; unconsciousness; convulsions; tremors; twitching; vomiting; blurred or double vision; drowsiness; dizziness; light-headedness; tinnitus; sensation of heat, cold or numbness; euphoria; apprehension; agitation; confused state; paresthesia; dysarthria.
Cardiovascular System: cardiovascular arrest; bradycardia which may lead to cardiac arrest; hypotension, Ventricular fibrillation, Ventricular tachycardia, Ventricular arrhythmia, Asystole.
Gastrointestinal Disorders: Hypoesthesia oral, Nausea.
Hematologic Effects: methemoglobinemia.
Psychiatric Disorders: Disorientation. - Drug Interactions:
- Pharmacodynamic Interactions:
- Digitalis derivatives: Monitor toxicity when lidocaine is used in patients with digitalis toxicity accompanied by supraventricular arrhythmia and/or atrioventricular block.
- When lidocaine is administered with other antiarrhythmic drugs such as amiodarone, phenytoin, procainamide, propranolol or quinidine, the cardiac effects may be additive or antagonistic and toxic effects may be additive.
- Pharmacokinetic Interactions:
- Concomitant treatment with drugs which are inhibitors of CYP1A2 and/or CYP3A4 has the potential to increase lidocaine plasma levels by decreasing lidocaine clearance and thereby prolonging the elimination half-life. Monitor toxicity when administering lidocaine with CYP1A2 and/or CYP3A4 inhibitors.
- Concomitant use of lidocaine at steady-state concentrations of the CYP1A2 inhibitor fluvoxamine increases intravenous lidocaine plasma AUC and Cmax and decreases MEGX AUC and Cmax. Monitor toxicity when co-administering these medications.
- Concomitant use of lidocaine with propofol, a hypnotic agent and CYP3A4 inhibitor, may increase lidocaine plasma levels by reducing lidocaine clearance. Monitor toxicity when co-administering lidocaine with propofol.
- Concomitant treatment with drugs which are inducers of CYP1A2 and/or CYP3A4 (e.g., phenytoin) has the potential to decrease lidocaine plasma levels and higher doses may be required.
- Concomitant use of lidocaine with a weak CYP1A2 and CYP3A4 inhibitor has been reported to increase lidocaine plasma levels and may result in toxic accumulation of the drug. Monitor toxicity when co-administering lidocaine with cimetidine.
- Beta-adrenergic blockers (e.g. propranolol): Concomitant use of lidocaine with beta-adrenergic blockers may increase lidocaine plasma levels by decreasing hepatic blood flow and thereby decrease lidocaine clearance. Monitor for toxicity when co-administering lidocaine with drugs that decrease hepatic blood flow.
Please see accompanying full Prescribing Information for Lidocaine Hydrochloride and 5% Dextrose Injection, USP.
Magnesium Sulfate in 5% Dextrose Injection, USP
1g/100 mL
Indications and Important Risk Information
Indications
Magnesium Sulfate in 5% Dextrose Injection is indicated for:
-
Prevention of eclampsia in patients with preeclampsia
-
Treatment of seizures and prevention of recurrent seizures in patients with eclampsia
Important Risk Information
Contraindications
Magnesium Sulfate in 5% Dextrose Injection is contraindicated in patients:
-
with heart block or myocardial damage
-
in diabetic coma
-
with myasthenia gravis
Warnings and Precautions
- Fetal-Neonatal Toxicity with Prolonged Use: Continuous administration of magnesium sulfate beyond 5 to 7 days in pregnant women can lead to hypocalcemia and bone abnormalities in the developing fetus, including skeletal demineralization and osteopenia. In addition, cases of neonatal fracture have been reported. Neonates of women receiving Magnesium Sulfate in 5% Dextrose Injection (especially with prolonged maternal use) are at risk for magnesium toxicity including hyporeflexia, hypotonia, and respiratory depression. There is one reported case of neonatal death as the result of magnesium toxicity after transplacental exposure. The shortest duration of magnesium sulfate treatment that can lead to fetal harm is not known.
- Risk of Magnesium Toxicity: Patients receiving Magnesium Sulfate in 5% Dextrose Injection are at risk for magnesium toxicity including respiratory depression, acute renal failure, and rarely, pulmonary edema. Monitor clinical signs of magnesium toxicity (for example, facial edema, diminished strength of deep tendon reflexes, respiratory depression) and magnesium concentrations during infusions. An injectable calcium salt should be immediately available to counteract the potential hazards of magnesium toxicity in patients with preeclampsia and eclampsia. If there is significant magnesium toxicity, stop the Magnesium Sulfate in 5% Dextrose Injection infusion and recheck serum magnesium concentration. Patients with renal impairment are at greater risk of magnesium toxicity because magnesium is excreted by the body solely by the kidneys. Urine output should be maintained at a level of 100 mL per 4 hours.
- Risk of Elevated Blood Glucose: Solutions containing dextrose should be used with caution in patients with known prediabetes or diabetes mellitus given the risk of elevated blood glucose.
- Co-administration with Unapproved Tocolytics: Do not use Magnesium Sulfate in 5% Dextrose Injection with unapproved tocolytics (e.g., beta-adrenergic agents such as terbutaline, or calcium channel blockers such as nifedipine). Serious adverse events including pulmonary edema and hypotension have occurred.
- Aluminum Toxicity: Magnesium Sulfate in 5% Dextrose Injection contains aluminum that may be toxic. Aluminum may reach toxic concentrations with prolonged parenteral administration in patients with renal impairment. Patients with renal impairment who receive parenteral concentrations of aluminum at greater than 4 to 5 mcg/kg/day accumulate aluminum at concentrations associated with central nervous system and bone toxicity. Tissue loading may occur at even lower rates of administration.
- Exacerbation of Myasthenia Gravis: Use of magnesium sulfate in patients with underlying myasthenia gravis can precipitate a myasthenic crisis. Myasthenic crisis is a life-threatening condition characterized by neuromuscular respiratory failure. Symptoms of myasthenic crisis may include difficulty swallowing, ptosis, facial droop, weakness, and/or difficulty breathing that may require intubation. If myasthenic crisis is suspected, discontinue use of Magnesium Sulfate in 5% Dextrose Injection immediately. Secure the patient’s airway.
- Adverse Reactions: The most common adverse reactions are flushing, sweating, hypotension, depressed reflexes, flaccid paralysis, hypothermia, circulatory collapse, cardiac and central nervous system (CNS) depression proceeding to respiratory paralysis, and hypocalcemia.
- Drug Interactions:
- Neuromuscular blocking agents (depolarizing and non-depolarizing): Potentiation and prolongation of neuromuscular blockade is possible with the concomitant use of Magnesium Sulfate in 5% Dextrose Injection
- Narcotics and/or propofol: Potentiation and prolongation of analgesia and CNS depression is possible with the concomitant use of Magnesium Sulfate in 5% Dextrose Injection
- Dihydropyridine calcium channel blockers: An exaggerated hypotensive response is possible with the concomitant use of Magnesium Sulfate in 5% Dextrose Injection
- Drugs that may induce magnesium loss with concomitant use of Magnesium Sulfate in 5% Dextrose Injection: Alcohol, aminoglycosides, amphotericin B, cisplatin, cyclosporine, digitalis, loop diuretics, and thiazide diuretics
Please see accompanying full Prescribing Information for Magnesium Sulfate in 5% Dextrose Injection, USP.
Magnesium Sulfate in Water for Injection
2 grams/50 mL, 4 grams/100 mL, 4 grams/50 mL
Indications and Important Risk Information
Indications
Magnesium Sulfate in Water for Injection is indicated for the prevention and control of seizures in preeclampsia and eclampsia, respectively. When used judiciously it effectively prevents and controls the convulsions of eclampsia without producing deleterious depression of the central nervous system of the mother or infant. However, other effective drugs are available for this purpose.
Important Risk Information
Contraindications
Intravenous magnesium should not be given to mothers with toxemia of pregnancy during the two hours preceding delivery.
Warnings and Precautions
- FETAL HARM: Continuous administration of magnesium sulfate beyond 5-7 days to pregnant women can lead to hypocalcemia and bone abnormalities in the developing fetus. These bone abnormalities include skeletal demineralization and osteopenia. In addition, cases of neonatal fracture have been reported. The shortest duration of treatment that can lead to fetal harm is not known. Magnesium sulfate should be used during pregnancy only if clearly needed.
Parenteral use in the presence of renal insufficiency may lead to magnesium intoxication. - Risk of Magnesium Toxicity: Because magnesium is removed from the body solely by the kidneys, the drug should be used with caution in patients with renal impairment. Urine output should be maintained at a level of 100 mL every four hours.
Monitoring serum magnesium levels and the patient's clinical status is essential to avoid the consequences of overdosage in toxemia. Clinical indications of a safe dosage regimen include the presence of the patellar reflex (knee jerk) and absence of respiratory depression (approximately 16 breaths or more/minute). An injectable calcium salt should be immediately available to counteract the potential hazards of magnesium intoxication in eclampsia.
Magnesium Sulfate in Water for Injection should be administered slowly to avoid producing hypermagnesemia. - Adverse Reactions: The adverse effects of parenterally administered magnesium usually are the result of magnesium intoxication. These include flushing, sweating, hypotension, depressed reflexes, flaccid paralysis, hypothermia, circulatory collapse, cardiac and central nervous system depression proceeding to respiratory paralysis. Hypocalcemia with signs of tetany secondary to magnesium sulfate therapy for eclampsia has been reported.
- Drug Interactions:
- Drug induced renal losses of magnesium occur with the following drugs or drug classes:
Alcohol, aminoglycosides, amphotericin B, cisplatin, cyclosporine, digitalis, and diuretics
Please see accompanying full Prescribing Information for Magnesium Sulfate in Water for Injection.
Milrinone Lactate in 5% Dextrose Injection
- 20 mg/100 mL (200 mcg/mL)
- 40 mg/200 mL (200 mcg/mL)
Indications and Important Risk Information
Indications
- Milrinone is indicated for the short-term intravenous treatment of patients with acute decompensated heart failure.
- There is no experience in controlled trials with infusions of milrinone for periods exceeding 48 hours.
Important Risk Information
- Contraindications: Milrinone is contraindicated in patients who are hypersensitive to it. Solutions containing dextrose may be contraindicated in patients with known allergy to corn or corn products.
- Use for Longer than 48 hours: Whether given orally or by continuous or intermittent intravenous infusion, based on a multicenter trial of 1088 patients with Class III and IV heart failure, milrinone has not been shown to be safe or effective in the longer (greater than 48 hours) treatment of patients with heart failure. In this study, patients with Class IV symptoms appeared to be at particular risk of life-threatening cardiovascular reactions. There is no evidence that milrinone given by longterm continuous or intermittent infusion does not carry a similar risk.
- Severe obstructive aortic or pulmonic valvular disease: Milrinone should not be used in patients with severe obstructive aortic or pulmonic valvular disease in lieu of surgical relief of the obstruction. Like other inotropic agents, it may aggravate outflow tract obstruction in hypertrophic subaortic stenosis.
- Monitoring: During therapy with milrinone, blood pressure and heart rate should be monitored and the rate of infusion slowed or stopped in patients showing excessive decreases in blood pressure.
- Acute Myocardial Infarction: No clinical studies have been conducted in patients in the acute phase of post myocardial infarction, therefore, milrinone is not recommended in these patients.
- Adverse Reactions: In clinical trials, adverse reactions included: ventricular arrhythmias (12.1%), ventricular ectopic activity (8.5%), supraventricular arrhythmias (3.8%), non-sustained ventricular tachycardia (2.8%), headaches (mild to moderate) (2.9%), hypotension (2.9%), angina/chest pain (1.2%), sustained ventricular tachycardia (1%), and ventricular fibrillation (0.2%). In the post-marketing experience, there have been rare cases of “torsades de pointes” reported.
- Drug Interactions: No clinical manifestations have been observed in limited experience with patients in whom milrinone was used concurrently with the following drugs: digitalis glycosides; lidocaine, quinidine; hydralazine, prazosin; isosorbide dinitrate, nitroglycerin; chlorthalidone, furosemide, hydrochlorothiazide, spironolactone; captopril; heparin, warfarin, diazepam, insulin; and potassium supplements.
- Pediatric Use: Safety and effectiveness in pediatric patients have not been established.
Please see accompanying full Prescribing Information for Milrinone Lactate in 5% Dextrose Injection.
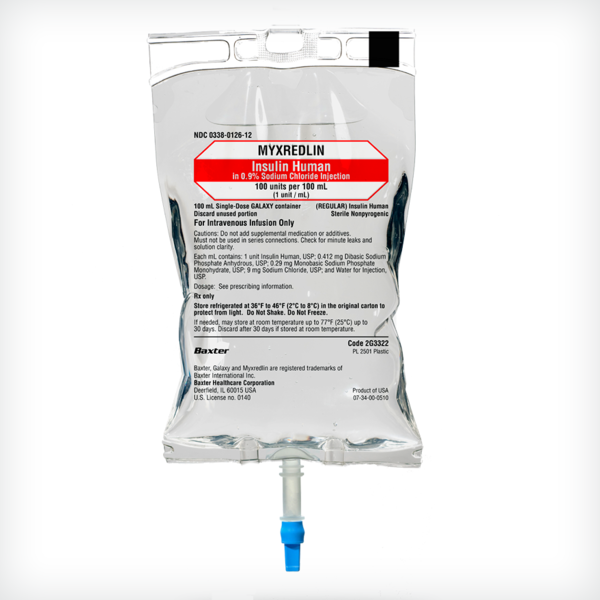
MYXREDLIN (Insulin Human) in 0.9% Sodium Chloride Injection
100 units per 100 mL (1 unit/mL)
Indications and Important Risk Information
Indications
MYXREDLIN is a short-acting human insulin indicated to improve glycemic control in adults and pediatric patients with diabetes mellitus.
Important Risk Information
Contraindications
-
During episodes of hypoglycemia
-
Hypersensitivity to insulin human or any of the excipients in MYXREDLIN
Warnings and Precautions
-
Hyper- or Hypoglycemia with Changes in Insulin Regimen: Carry out under close medical supervision and increase frequency of blood glucose monitoring.
-
Administer MYXREDLIN intravenously ONLY under medical supervision with close monitoring of blood glucose and potassium levels. Hypokalemia may be life-threatening if not treated.
-
Individualize dose based on metabolic needs, blood glucose monitoring results, and glycemic control goal. Dosage adjustments may be needed with changes in nutrition, renal, or hepatic function or during acute illness.
-
Adverse reactions observed with insulin human injection include hypoglycemia, allergic reactions, weight gain and edema.
-
Fluid Retention and Heart Failure with Concomitant Use of Thiazolidinediones (TZDs): Observe for signs and symptoms of heart failure; such as shortness of breath, swelling of your ankles or feet, or sudden weight gain.
Dosage and Administration
-
Inspect MYXREDLIN visually before use. It should appear clear and colorless. Do not use MYXREDLIN if particulate matter or coloration is seen.
-
Do not add supplementary medication or additives.
-
Do not use in series connections.
-
Do not shake or freeze. Discard unused portion.
Please see accompanying full Prescribing Information for MYXREDLIN (Insulin Human) in 0.9% Sodium Chloride Injection.
NEXTERONE (amiodarone HCl) Premixed Injection
150 mg/100 mL and 360 mg/200 mL
Indications and Important Risk Information
Indications
NEXTERONE Premixed Injection is indicated for initiation of treatment and prophylaxis of frequently recurring ventricular fibrillation (VF) and hemodynamically unstable ventricular tachycardia (VT) in patients refractory to other therapy.
Important Risk Information
- Contraindications: NEXTERONE Premixed Injection is contraindicated in patients with:
- Known hypersensitivity to any of the components of NEXTERONE Premixed Injection, including iodine
- Cardiogenic shock
- Marked sinus bradycardia
- Second- or third-degree atrioventricular (AV) block unless a functioning pacemaker is available
- Persistence of Adverse Effects: Because of the long half-life of amiodarone (9 to 36 days) and its metabolite desethylamiodarone (9 to 30 days), adverse reactions or interactions, as well as observed adverse effects, can persist following amiodarone withdrawal.
- Hypotension: Most often seen in the first several hours of treatment and likely related to the rate of infusion. In some cases, hypotension may be refractory and result in a fatal outcome. To treat: Slow the infusion; as needed, add vasopressor drugs, positive inotropic agents, and volume expansion.
- Primary Graft Dysfunction (PGD) Post Cardiac Transplant: In retrospective studies, amiodarone use in transplant recipients prior to heart transplants has been associated with an increased risk of PGD. Severe PGD may be irreversible. For patients who are on the heart transplant waiting list, consideration should be given to use an alternative antiarrhythmic drug as early as possible before transplant.
- Bradycardia and Atrioventricular Block: May require slowing the infusion rate or discontinuing NEXTERONE Premixed Injection. In some patients, inserting a pacemaker is required. Have a temporary pacemaker available when treating a patient predisposed to bradycardia or AV block.
- Hepatic Injury: Acute hepatocellular necrosis leading to hepatic coma, acute renal failure, and death has been associated. Intravenous infusions at much higher concentrations and rates of infusion than those recommended appear to increase this risk. Carefully monitor patients receiving NEXTERONE Premixed Injection for evidence of progressive hepatic injury. Consider reducing the rate of administration or withdrawing NEXTERONE Premixed Injection if hepatic injury occurs.
- Proarrhythmia: NEXTERONE Premixed Injection may cause a worsening of existing arrhythmias or precipitate a new arrhythmia, sometimes leading to fatal outcomes. Proarrhythmia, primarily torsade de pointes (TdP), has been associated with prolongation by intravenous amiodarone. Monitor patients for QTc prolongation during infusion. Reserve the combination of amiodarone with other antiarrhythmic therapies that prolong the QTc to patients with life-threatening ventricular arrhythmias who are incompletely responsive to a single agent. Correct hypokalemia, hypomagnesemia or hypocalcemia whenever possible before initiating treatment.
- Pulmonary Injury: There have been post-marketing reports of acute-onset (days to weeks) pulmonary injury. Some cases have progressed to respiratory failure or death. Monitor for new respiratory symptoms and evaluate appropriately. Obtain a baseline chest X-ray and pulmonary function tests in patients who are expected to be receiving amiodarone chronically.
- Loss of Vision: Cases of optic neuropathy and optic neuritis, usually resulting in visual impairment, have been reported. In some cases, visual impairment has progressed to permanent blindness. Optic neuropathy and neuritis may occur at any time following initiation of therapy. Perform an ophthalmic examination if symptoms of visual impairment appear. Reevaluate the necessity of amiodarone therapy if optic neuropathy or neuritis is suspected.
- Thyroid Abnormalities: NEXTERONE Premixed Injection inhibits peripheral conversion of thyroxine (T4) to triiodothyronine (T3) and may cause increased T3 levels, and increased levels of inactive reverse T3 (rT3) in clinically euthyroid patients. Monitor thyroid function prior to treatment and periodically thereafter, particularly in elderly patients, and in any patient with a history of thyroid nodules, goiter, or other thyroid dysfunction. Hyperthyroidism may induce arrhythmia breakthrough. If any new signs of arrhythmia appear, the possibility of hyperthyroidism should be considered.
- Neonatal Injury: Amiodarone can cause fetal harm when administered to a pregnant woman. Fetal exposure may increase the potential for adverse experiences including cardiac, thyroid, neurodevelopmental, neurological and growth effects in neonate. Inform the patient of the potential hazard to the fetus if NEXTERONE Premixed Injection is administered during pregnancy or if the patient becomes pregnant while taking.
- Hypersensitivity Reactions: Anaphylactic/anaphylactoid reactions have been reported including shock (sometimes fatal), cardiac arrest, and the following manifestations: hypotension, tachycardia, hypoxia, cyanosis, rash, Stevens-Johnson syndrome, flushing, hyperhidrosis and cold sweat.
- Adverse Reactions: The most common adverse reactions (1-2%) leading to discontinuation of intravenous amiodarone therapy are hypotension, asystole/cardiac arrest/pulseless electrical activity, VT, and cardiogenic shock. Other important adverse reactions are torsade de pointes, congestive heart failure, and liver function test abnormalities.
- Drug Interactions: Amiodarone is a substrate for CYP3A and CYP2C8, so inhibitors and inducers affect amiodarone exposure. Amiodarone inhibits p-glycoprotein and CYP1A2, CYP2C9, CYP2D6, and CYP3A, increasing exposure to other drugs.
Please see accompanying full Prescribing Information for NEXTERONE (amiodarone HCl) Premixed Injection.
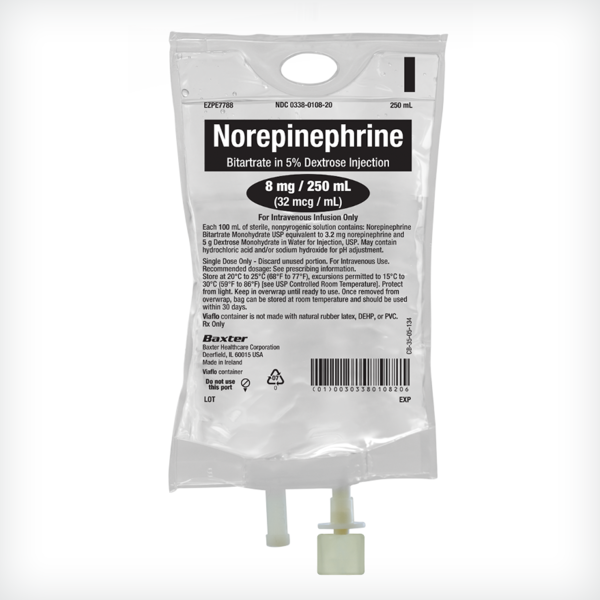
Norepinephrine Bitartrate in 5% Dextrose Injection
4 mg equivalent of norepinephrine (16 mcg/mL) in 5% dextrose 250 mL
8 mg equivalent of norepinephrine (32 mcg/mL) in 5% dextrose 250 mL
16 mg equivalent of norepinephrine (64 mcg/mL) in 5% dextrose 250 mL
Indication and Important Risk Information
Indication
• Norepinephrine Bitartrate in Dextrose Injection is indicated to raise blood pressure in adult patients with severe, acute hypotension.
Important Risk Information
- Contraindications: None.
- Tissue Ischemia: Administration of Norepinephrine Bitartrate in Dextrose Injection to patients who are hypotensive from hypovolemia can result in severe peripheral and visceral vasoconstriction, decreased renal perfusion and reduced urine output, tissue hypoxia, lactic acidosis, and reduced systemic blood flow despite “normal” blood pressure. Address hypovolemia prior to initiating Norepinephrine Bitartrate in Dextrose Injection. Avoid use in patients with mesenteric or peripheral vascular thrombosis, as this may increase ischemia and extend the area of infarction.
Gangrene of the extremities has occurred in patients with occlusive or thrombotic vascular disease or who received prolonged or high dose infusions. Monitor for changes to the skin of the extremities in susceptible patients.
Extravasation of Norepinephrine Bitartrate in Dextrose Injection may cause necrosis and sloughing of surrounding tissue. To reduce the risk of extravasation, infuse into a large vein, check the infusion site frequently for free flow, and monitor for signs of extravasation.
Avoid administration into the veins in the leg in elderly patients.
Emergency Treatment of Extravasation: Infiltrate the ischemic area as soon as possible, using a syringe with a fine hypodermic needle with 5 to 10 mg of phentolamine mesylate in 10 to 15 mL of 0.9% Sodium Chloride Injection in adults. - Hypotension after Abrupt Discontinuation: Sudden cessation of the infusion rate may result in marked hypotension. When discontinuing the infusion, gradually reduce the infusion rate while expanding blood volume with intravenous fluids.
- Cardiac Arrhythmias: Norepinephrine Bitartrate in Dextrose Injection elevates intracellular calcium concentrations and may cause arrhythmias, particularly in the setting of hypoxia or hypercarbia. Perform continuous cardiac monitoring of patients with arrhythmias.
- Elderly Patients: May be at a greater risk of developing adverse reactions.
- Adverse Reactions: Most common adverse reactions are hypertension and bradycardia.
- Drug Interactions:
- Co-administration of Norepinephrine Bitartrate in Dextrose Injection with monoamine oxidase (MAO) inhibitors or other drugs with MAO-inhibiting properties (e.g., linezolid) or with tricyclic antidepressants can cause severe, prolonged hypertension.
- Anti-diabetics: Norepinephrine Bitartrate in Dextrose Injection can decrease insulin sensitivity and raise blood glucose.
- Concomitant use of Norepinephrine Bitartrate in Dextrose Injection with halogenated anesthetics may lead to ventricular tachycardia or ventricular fibrillation. Monitor cardiac rhythm in patients receiving concomitant halogenated anesthetics.
Please see accompanying full Prescribing Information for Norepinephrine Bitartrate in 5% Dextrose Injection.
Potassium Chloride Injection (Highly Concentrated)
- 10mEq/ 100mL, 20mEq/ 100mL, 40mEq/ 100mL
- 10mEq/ 50mL, 20mEq/ 50mL
Indications and Important Risk Information
Indications
- Potassium Chloride Injection is indicated in the treatment of potassium deficiency states when oral replacement is not feasible.
- Highly-concentrated, ready-to-use Potassium Chloride Injection is intended for the maintenance of serum potassium levels and potassium supplementation in fluid restricted patients.
- When using these products, these patients should be on continuous cardiac monitoring and frequent testing for serum potassium concentration and acid-base balance. To avoid potassium intoxication, do not infuse these solutions rapidly.
Important Risk Information
- Contraindications: Potassium Chloride Injection is contraindicated in patients with hyperkalemia and patients with known hypersensitivity to Potassium Chloride Injection.
- Hyperkalemia: Potassium Chloride Injection should be administered with extreme caution, if at all, to patients with conditions predisposing to hyperkalemia and/or associated with increased sensitivity to potassium. Administration of concentrated potassium solutions can cause cardiac conduction disorders (including complete heart block) and other cardiac arrhythmias at any time during infusion. Frequently, mild or moderate hyperkalemia is asymptomatic and may be manifested only by increased serum potassium concentrations and, possibly, characteristic EKG changes. However, fatal arrhythmias can develop at any time during hyperkalemia.
- Tissue Damage and Thrombophlebitis: When infusing concentrated potassium solutions, including Potassium Chloride Injection, care must be taken to prevent paravenous administration or extravasation because such solutions may be associated with tissue damage, which may be severe and include vascular, nerve, and tendon damage, leading to surgical intervention, including amputation. Secondary complications including pulmonary embolism from thrombophlebitis have been reported. Administer intravenously only with a calibrated infusion device at a slow, controlled rate. Whenever possible, administration via a central route is recommended for all concentrations of Potassium Chloride Injection. Correct placement of the catheter should be verified before administration.
- Hyponatremia: Potassium Chloride Injection may cause hyponatremia. The risk for hyponatremia is increased, in pediatric patients, elderly patients, postoperative patients, those with psychogenic polydipsia and in patients treated with medications that increase the risk of hyponatremia. Avoid Potassium Chloride Injection in patients with or at risk for hyponatremia. Acute hyponatremia can lead to acute hyponatremic encephalopathy characterized by headache, nausea, seizures, lethargy and vomiting. Patients with brain edema are at particular risk of severe, irreversible, and life-threatening brain injury. Monitoring of serum sodium is particularly important for hypotonic fluids. Potassium Chloride Injection has an osmolarity of 200 to 799 mOsmol/L.
- Fluid Overload: Depending on the volume and rate of infusion, and the patient’s underlying clinical condition, the intravenous administration of Potassium Chloride Injection can cause electrolyte disturbances such as overhydration/hypervolemia and congested states including central and peripheral edema. Avoid Potassium Chloride Injection in patients with or at risk for fluid and/or solute overloading. If use cannot be avoided, monitor fluid balance, electrolyte concentrations and acid base balance.
- Hyperchloremia: In patients with or at risk of hyperchloremia, Potassium Chloride Injection may exacerbate or result in hyperchloremia. Monitor plasma chloride levels and renal function in such patients.
- Adverse Reactions: Post marketing adverse reactions (not noted elsewhere) included: Cardiac arrest, asystole, ventricular fibrillation, bradycardia as a manifestation of rapid intravenous administration and/or of hyperkalemia; Dyspnea; Chest pain; Infusion reactions including: infusion site thrombosis, infusion site phlebitis, infusion site erythema, infusion site swelling, infusion site pain, infusion site irritation, and/or a burning sensation.
- Drug interactions: Administration of Potassium Chloride Injection in patients treated concurrently or recently with other products that can cause hyperkalemia or increase the risk of hyperkalemia, increases the risk of severe and potentially fatal hyperkalemia, particularly in the presence of other risk factors for hyperkalemia. If use cannot be avoided, monitor serum potassium concentrations.
Administration of Potassium Chloride Injection in patients treated concomitantly with drugs associated with hyponatremia may increase the risk of developing hyponatremia. If use cannot be avoided, monitor serum sodium concentrations. - Pediatric Use: These products should not be used in children at this time. Safety and effectiveness of Potassium Chloride Injection in pediatric patients have not been established by adequate and well-controlled studies.
Please see accompanying full Prescribing Information for Potassium Chloride Injection.
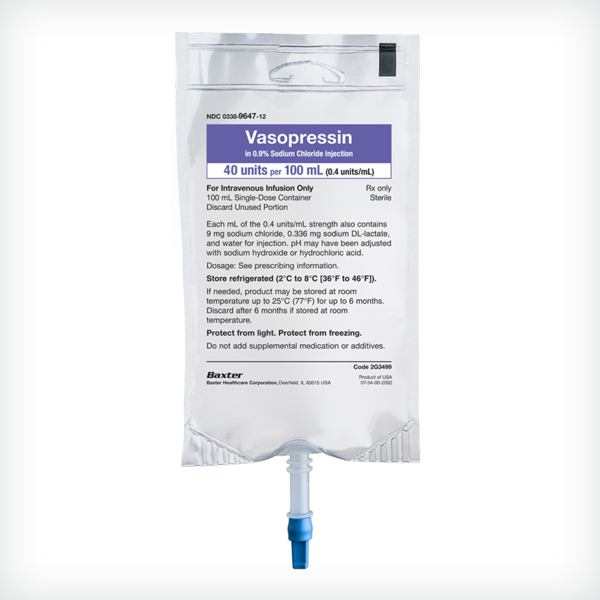
Vasopressin in 0.9% 100 mL Sodium Chloride Injection
20 units vasopressin (0.2 units/mL) in 0.9% sodium chloride
40 units vasopressin (0.4 units/mL) in 0.9% sodium chloride
Indications and Important Risk Information
Indications
- Vasopressin in Sodium Chloride Injection is indicated to increase blood pressure in adults with vasodilatory shock who remain hypotensive despite fluids and catecholamines.
Important Risk Information
- Contraindications: Vasopressin in Sodium Chloride Injection is contraindicated in patients with a known allergy or hypersensitivity to 8-L-arginine vasopressin.
- Worsening Cardiac Function: A decrease in cardiac index may be observed with the use of vasopressin.
- Reversible Diabetes Insipidus: Patients may experience reversible diabetes insipidus, manifested by the development of polyuria, a dilute urine, and hypernatremia, after cessation of treatment with vasopressin. Monitor serum electrolytes, fluid status, and urine output after vasopressin discontinuation. Some patients may require readministration of vasopressin or administration of desmopressin to correct fluid and electrolyte shifts.
- Adverse Reactions:
– The most common adverse reactions include decreased cardiac output, bradycardia, tachyarrhythmias, hyponatremia, and ischemia (coronary, mesenteric, skin, digital). - Drug Interactions:
– Pressor effects of catecholamines and Vasopressin in Sodium Chloride Injection are expected to be additive.
– Indomethacin may prolong effects of Vasopressin in Sodium Chloride Injection.
– Co-administration of ganglionic blockers or drugs causing SIADH (syndrome of inappropriate antidiuretic hormone secretion) may increase the pressor response.
– Co-administration of drugs causing diabetes insipidus may decrease the pressor response. - Pregnancy: May induce tonic uterine contractions that could threaten the continuation of pregnancy.
Please see accompanying full Prescribing Information for Vasopressin in 0.9% Sodium Chloride Injection.